FDA launches safety review of two RSV drugs for infants as RFK Jr. scrutinizes immunizations
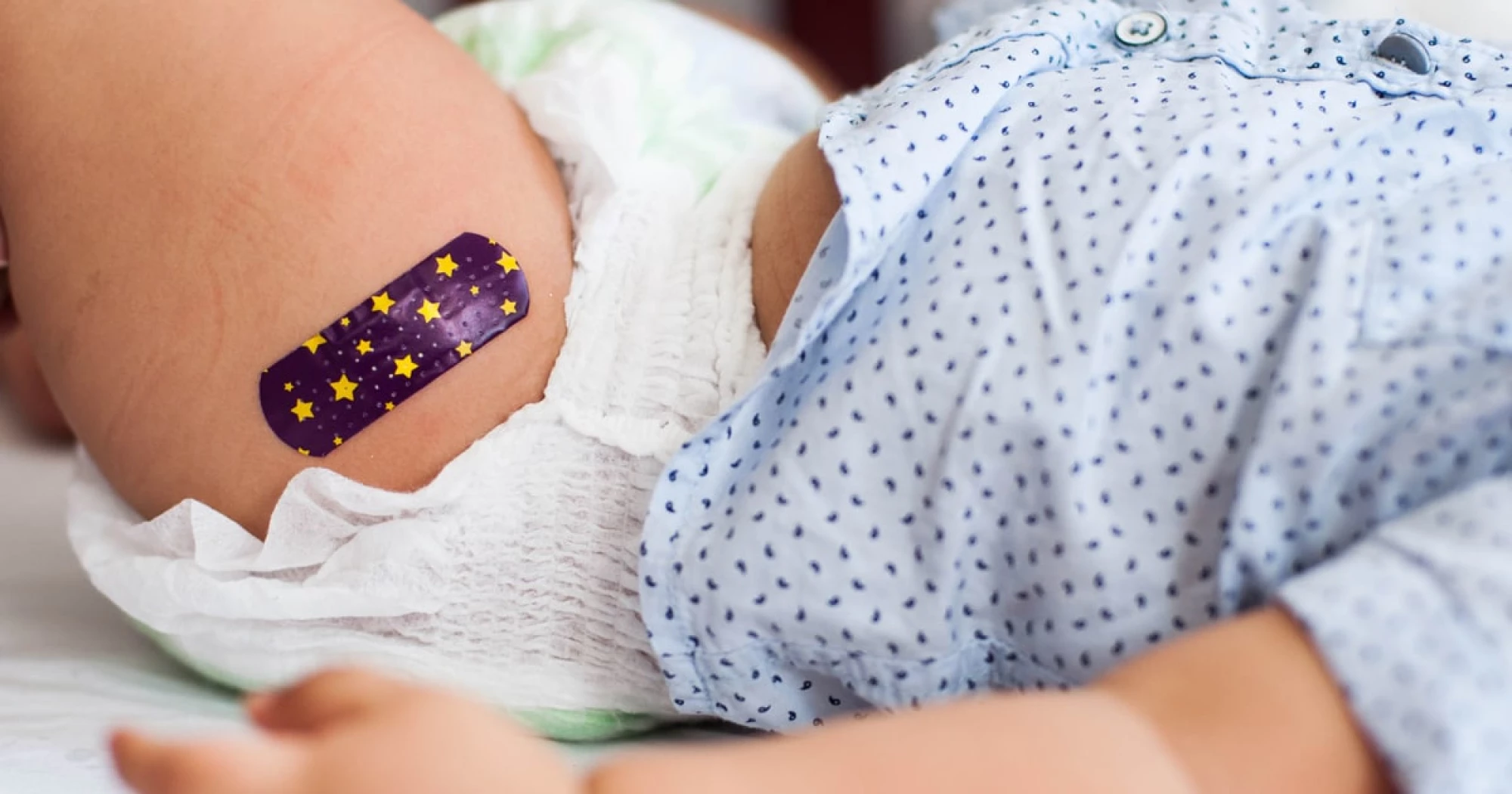
The Food and Drug Administration has launched a safety review of two approved RSV drugs for infants, the latest immunizations to face scrutiny under Health Secretary RFK, Jr.
The Food and Drug Administration has launched a safety review of two approved RSV drugs for infants, the latest immunizations to face scrutiny under Health Secretary Robert F. Kennedy Jr.
No safety issues have been reported with either of the respiratory syncytial virus drugs: Beyfortus, from Sanofi and AstraZeneca, and Enflonsia, from Merck.
Andrew Nixon, a spokesperson at the Department of Health and Human Services, said in an emailed statement that the FDA is “rigorously reviewing the available data, as it does for all products, to ensure decisions remain rooted in evidence-based science and in the best interest of patients.”
“FDA routinely evaluates emerging safety information and will update product labeling if warranted by the totality of the evidence,” Nixon said.
A Sanofi spokesperson said in an email that the safety and effectiveness of Beyfortus, a monoclonal antibody drug, has been demonstrated in over 50 clinical studies and real-world studies, involving more than 400,000 infants.
Rating: 5